Bioavailable Nutrition: A New Approach to an Old Problem
Introduction
In order to achieve high and quality yields, proper nutritional management is necessary. This requires considering the soil-plant-fruit relationship and understanding the availability of nutrients in the soil to provide the necessary amounts of micro and macronutrients.
Micronutrients play a crucial role in crop development, even if they are required in minimal quantities. Deficiencies in certain microelements can have a significant impact, causing reductions in production yields as well as in crop quality.
In plant nutrition, chelation or complexation is used to prevent the elements to be incorporated into the plant from precipitating in the soil or extracellular medium.
Chelates are complex compounds characterized by binding to metal ions through covalent bonds, creating a ring. These molecules envelop the mineral, preventing unfavorable interactions such as oxidation, immobilization, and precipitation, all of which make the mineral inaccessible to the plant. In contrast, complexes have a weaker bond, but this facilitates the release of nutrients for subsequent assimilation.
These compounds can be generally classified as synthetic chelates, such as ethylenediaminetetraacetic acid (EDTA), ethylenediaminedihydroxyphenylacetic acid (EDDHA), or natural complexing agents such as amino acids, organic acids, lignin, etc.
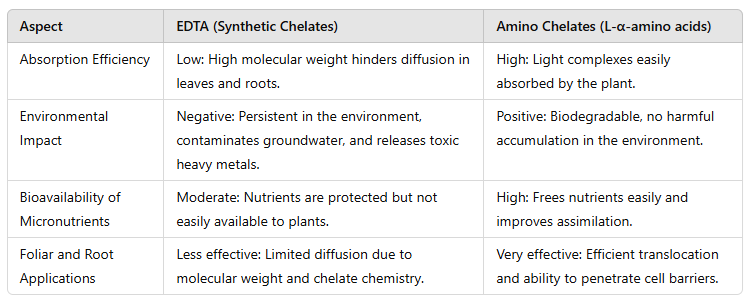
Synthetic chelates have been key to the nutrition of cropping systems. However, their low absorption efficiency by the plant has led to high application rates, resulting in high costs for farmers and significant environmental problems.
Currently, according to Beltyukova et al. (2023) Suitable chelating agents for nutrient supply must meet: 1) high biodegradability, 2) low ecotoxicity of the starting compounds and their biodegrading metabolites, 3) high bioavailability to provide macro- and microelements to crops, and 4) efficient transport of microelements to plant cells.
In this sense, L-α-amino acids are organic molecules naturally present in biological systems, making them safe and readily biodegradable in the environment. They have a medium complexing capacity, which reduces the precipitation of elements in the soil, and is suitable for incorporation into the plant via soil and especially through foliar application.
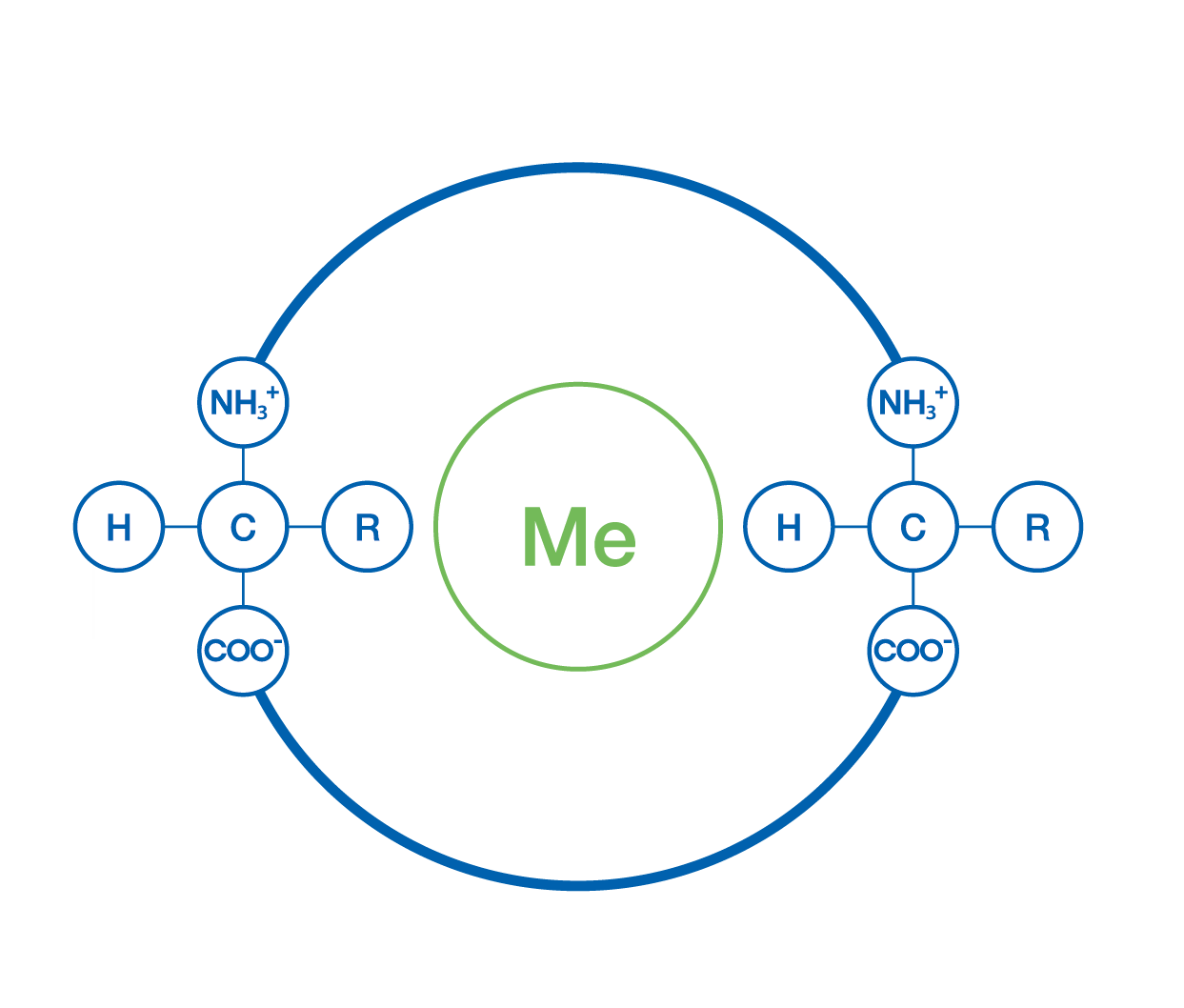
Efficient absorption by the plant
Microelements or metals bind to L-α-amino acids through the carboxyl group with one or more bonds, forming a complex that is easily absorbed and translocated within the plant, compared to inorganic salts or synthetic chelates, which have difficulty diffusing from the leaf surface to the plant interior due to their high molecular weight.
Furthermore, microelements complexed with complex mixtures of L-α-amino acids are especially effective when applied foliarly, as the amino acid mixture exhibits different polarities and can cross both lipophilic and hydrophilic barriers.
Once inside the plant, complexes with amino acids applied both foliarly and root-derived are recognized by specific transporters in cell membranes, which modulate membrane permeability and promote ion transport across them.
Double action: complexation and biostimulation
Amino acid-based complexes, in addition to promoting the absorption and translocation of micronutrients within the plant, exert a biostimulating effect.
Applications of L-α-amino acids complement the amino acids synthesized by the plant itself, representing a significant saving in metabolic energy during periods of stress. Furthermore, the exogenous application of amino acids has proven effective in stimulating multiple physiological processes, promoting crop growth, yield, and quality.
Environmental impact
Synthetic chelates have historically been the way to correct micronutrient deficiencies in crops. However, it is now known that molecules such as EDTA degrade very slowly and, as a result, accumulate in groundwater, surface water, and soil.
Specifically in soil, EDTA, being an acid, reduces the pH and promotes the desorption of heavy metals (copper, zinc, cadmium, chromium), converting them into soluble forms and making them available for plant absorption, which contributes to their transfer along food chains. Furthermore, recent studies have shown that EDTA is capable of destroying the outer membranes of some beneficial soil bacteria.
For all these reasons, the use of EDTA chelates is currently severely restricted in several countries.
Comparative Table between Synthetic Chelates and Aminochelates:
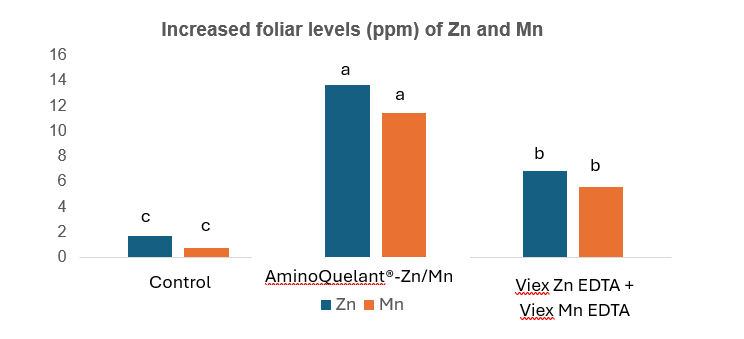
Zinc (Zn), manganese (Mn) and boron (B) aminochelates
Zinc, manganese, and boron play an essential role in plant growth and development, impacting multiple physiological processes.
Traditionally, the chelates used to treat deficiencies of these micronutrients have been primarily EDTA and DTPA-based, and in the case of boron, boric acid, borax, or boron ethanolamine.
For these micronutrients, the effectiveness of their combined application with L-α-amino acids under different abiotic stress conditions is notable, with a superior effect on crop yield and quality compared to synthetic chelates.

Image 2. Symptoms of zinc deficiency mainly.
Specifically, zinc and boron deficiencies reduce protein synthesis in the plant, which is reactivated by the application of these microelements complexed with amino acids.
Zinc deficiency significantly reduces stomatal opening, so the combined application of L-α-amino acids and zinc will stimulate opening, helping the plant recover.
Another example is the damage to cell membranes caused by deficiency stress, which is mitigated by the application of boron and zinc amino acid complexes.

